Sulfonyl Halides: Versatile Compounds in Organic Chemistry
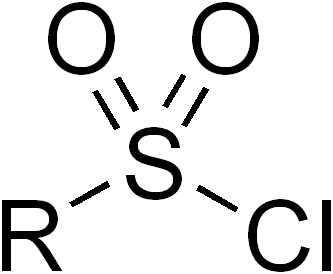
Sulfonyl halides, also known as sulfonyl halides, are a class of organic compounds that play a significant role in modern organic synthesis. These compounds contain a sulfonyl group (R-SO2-) bonded to a halogen atom (X), where X can be chlorine, bromine, or iodine. Sulfonyl halides are highly reactive and versatile, making them valuable building blocks for the construction of various organic molecules. In this article, we will explore the properties, synthesis, and applications of sulfonyl halides in organic chemistry.
Properties and Synthesis: Sulfonyl halides are characterized by the presence of a polarized sulfonyl group, which imparts high reactivity to these compounds. The electronegative halogen atom enhances the electrophilicity of the sulfonyl carbon, making it prone to nucleophilic attack. The synthesis of sulfonyl halides typically involves the reaction of a sulfonic acid with a halogenating agent, such as phosphorus pentahalide (PCl5, PBr5) or thionyl chloride (SOCl2). These reactions result in the replacement of a hydroxyl group (-OH) in the sulfonic acid with a halogen atom.
Reactivity and Functional Group Interconversion: Sulfonyl halides are powerful electrophiles and react readily with various nucleophiles, such as amines, alcohols, thiols, and organometallic reagents. The nucleophilic attack on the sulfonyl carbon leads to the formation of new covalent bonds and allows for the introduction of diverse functional groups. For example, reaction with a primary amine results in the formation of a sulfonamide, while reaction with an alcohol leads to the formation of a sulfonate ester. Moreover, sulfonyl halides can undergo substitution reactions with nucleophilic aromatic compounds, enabling the introduction of sulfonamide or sulfonate groups into aromatic rings.
Applications in Organic Synthesis: The versatile reactivity of sulfonyl halides makes them valuable intermediates in organic synthesis. One of the key applications of sulfonyl halides is in the synthesis of pharmaceuticals. Many drug molecules contain sulfonamide or sulfonate moieties, which can be conveniently introduced using sulfonyl halides. These functional groups often contribute to the desired biological activity of the drugs. Additionally, sulfonyl halides find extensive use in the synthesis of agrochemicals, dyes, and other fine chemicals.
Another important application of sulfonyl halides is in the construction of heterocycles. By reacting with nucleophiles bearing appropriate functional groups, sulfonyl halides can participate in cyclization reactions to form various heterocyclic systems. These heterocycles serve as the structural core of many natural products and biologically active compounds. The ability to rapidly construct these frameworks using sulfonyl halides provides chemists with powerful tools for the synthesis of complex organic molecules.
Furthermore, sulfonyl halides have found utility in materials science. They can be used as crosslinking agents or reactive intermediates for the modification of polymers. The presence of the sulfonyl group imparts enhanced solubility, stability, and functionality to the resulting materials. This versatility has led to the development of novel materials with unique properties for applications ranging from coatings to electronics.
In conclusion, sulfonyl halides are versatile and valuable compounds in organic chemistry. Their reactivity allows for the introduction of various functional groups, making them indispensable building blocks in the synthesis of pharmaceuticals, agrochemicals, and other fine chemicals.
293
0
0


Comments
All Comments (0)